Mitomycin-C is the chemotherapy agent commonly used during HIPEC (hyperthermic intraperitoneal chemotherapy) following cytoreductive surgery across the world. The main other chemotherapy option is Oxaliplatin.
Mitomycin-C is almost exclusively supplied by Kyowa Kirin. This company have identified concerns regarding the quality control and manufacturing process of Mitomycin-C. All current stock has been recalled.
The CAS alert states that there is no date available to advise when stock will be available. The Royal College of Opthalmologists website suggest that stock may be available again by the end of January 2020. There are other potential supply routines for Mitomycin-C and the Deptartment of Health have provided these to healthcare providers.
This supply issue has the potential to impact on patients due for surgery and HIPEC in the next few months, although I believe healthcare providers will be working hard to find alternative sources of intra peritoneal chemotherapy for patients. If you have any concerns then I suggest you discuss this with your Clinical Nurse Specialists for more information.
Georgina
Dr Georgina Morgan MB ChB, Trustee
What are the statistics for pseudomyxoma peritonei?
It’s estimated that 1 in 2 people in the UK born after 1960 will be diagnosed with some form of cancer during their lifetime. What numbers are there for pseudomyxoma peritonei (PMP)?
BromAc trial in Spain
Dr Arona Sanchez from Cordoba tells us about an exciting new trial for patients experiencing a recurrence of PMP.
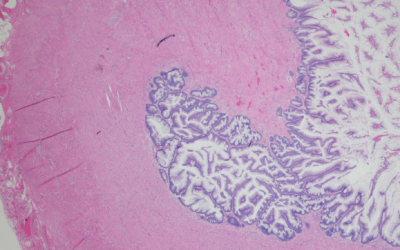
Photo credit: Hellerhoff, CC BY-SA 3.0, via Wikimedia Commons
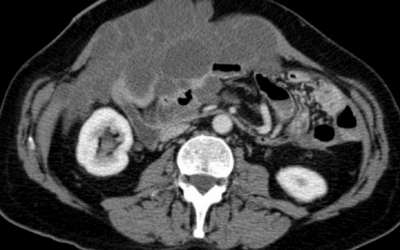
The Biology of pseudomyxoma peritonei (PMP)
Pseudomyxoma Survivor is supporting exciting new research into the biology of pseudomyxoma peritonei.